nIXS $L_{2,3}$
Besides low energy transitions nIXS can be used as a core level spectroscopy technique. One then measures resonances with non-resonant inelastic x-ray scattering 
This tutorial uses Radial wave functions in order to calculate the length of q dependence. You can download them in a zip file here nio_data.zip. Please unpack this file and make sure to have the folders NiO_Experiment and NiO_Radial in the same folder as you do the calculations.
The input script:
- NIXS_L23.Quanty
-- using inelastic x-ray scattering one can not only measure low energy excitations, -- but equally well core to core transitions. This allows one to probe for example -- 3p to 3d transitions using octupole operators. -- We use the definitions of all operators and basis orbitals as defined in the file -- include and can afterwards directly continue by creating the Hamiltonian -- and calculating the spectra dofile("Include.Quanty") -- The parameters and scheme needed is the same as the one used for XAS -- We follow the energy definitions as introduced in the group of G.A. Sawatzky (Groningen) -- J. Zaanen, G.A. Sawatzky, and J.W. Allen PRL 55, 418 (1985) -- for parameters of specific materials see -- A.E. Bockquet et al. PRB 55, 1161 (1996) -- After some initial discussion the energies U and Delta refer to the center of a configuration -- The L^10 d^n configuration has an energy 0 -- The L^9 d^n+1 configuration has an energy Delta -- The L^8 d^n+2 configuration has an energy 2*Delta+Udd -- -- If we relate this to the onsite energy of the L and d orbitals we find -- 10 eL + n ed + n(n-1) U/2 == 0 -- 9 eL + (n+1) ed + (n+1)n U/2 == Delta -- 8 eL + (n+2) ed + (n+1)(n+2) U/2 == 2*Delta+U -- 3 equations with 2 unknowns, but with interdependence yield: -- ed = (10*Delta-nd*(19+nd)*U/2)/(10+nd) -- eL = nd*((1+nd)*Udd/2-Delta)/(10+nd) -- -- For the final state we/they defined -- The 2p^5 L^10 d^n+1 configuration has an energy 0 -- The 2p^5 L^9 d^n+2 configuration has an energy Delta + Udd - Upd -- The 2p^5 L^8 d^n+3 configuration has an energy 2*Delta + 3*Udd - 2*Upd -- -- If we relate this to the onsite energy of the p and d orbitals we find -- 6 ep + 10 eL + n ed + n(n-1) Udd/2 + 6 n Upd == 0 -- 6 ep + 9 eL + (n+1) ed + (n+1)n Udd/2 + 6 (n+1) Upd == Delta -- 6 ep + 8 eL + (n+2) ed + (n+1)(n+2) Udd/2 + 6 (n+2) Upd == 2*Delta+Udd -- 5 ep + 10 eL + (n+1) ed + (n+1)(n) Udd/2 + 5 (n+1) Upd == 0 -- 5 ep + 9 eL + (n+2) ed + (n+2)(n+1) Udd/2 + 5 (n+2) Upd == Delta+Udd-Upd -- 5 ep + 8 eL + (n+3) ed + (n+3)(n+2) Udd/2 + 5 (n+3) Upd == 2*Delta+3*Udd-2*Upd -- 6 equations with 3 unknowns, but with interdependence yield: -- epfinal = (10*Delta + (1+nd)*(nd*Udd/2-(10+nd)*Upd) / (16+nd) -- edfinal = (10*Delta - nd*(31+nd)*Udd/2-90*Upd) / (16+nd) -- eLfinal = ((1+nd)*(nd*Udd/2+6*Upd)-(6+nd)*Delta) / (16+nd) -- -- -- -- note that ed-ep = Delta - nd * U and not Delta -- note furthermore that ep and ed here are defined for the onsite energy if the system had -- locally nd electrons in the d-shell. In DFT or Hartree Fock the d occupation is in the end not -- nd and thus the onsite energy of the Kohn-Sham orbitals is not equal to ep and ed in model -- calculations. -- -- note furthermore that ep and eL actually should be different for most systems. We happily ignore this fact -- -- We normally take U and Delta as experimentally determined parameters -- number of electrons (formal valence) nd = 8 -- parameters from experiment (core level PES) Udd = 7.3 Upd = 8.5 Delta = 4.7 -- parameters obtained from DFT (PRB 85, 165113 (2012)) F2dd = 11.14 F4dd = 6.87 F2pd = 6.67 G1pd = 4.92 G3pd = 2.80 tenDq = 0.56 tenDqL = 1.44 Veg = 2.06 Vt2g = 1.21 zeta_3d = 0.081 zeta_2p = 11.51 Bz = 0.000001 H112 = 0 ed = (10*Delta-nd*(19+nd)*Udd/2)/(10+nd) eL = nd*((1+nd)*Udd/2-Delta)/(10+nd) epfinal = (10*Delta + (1+nd)*(nd*Udd/2-(10+nd)*Upd)) / (16+nd) edfinal = (10*Delta - nd*(31+nd)*Udd/2-90*Upd) / (16+nd) eLfinal = ((1+nd)*(nd*Udd/2+6*Upd) - (6+nd)*Delta) / (16+nd) F0dd = Udd + (F2dd+F4dd) * 2/63 F0pd = Upd + (1/15)*G1pd + (3/70)*G3pd Hamiltonian = F0dd*OppF0_3d + F2dd*OppF2_3d + F4dd*OppF4_3d + zeta_3d*Oppldots_3d + Bz*(2*OppSz_3d + OppLz_3d) + H112 * (OppSx_3d+OppSy_3d+2*OppSz_3d)/sqrt(6) + tenDq*OpptenDq_3d + tenDqL*OpptenDq_Ld + Veg * OppVeg + Vt2g * OppVt2g + ed * OppN_3d + eL * OppN_Ld XASHamiltonian = F0dd*OppF0_3d + F2dd*OppF2_3d + F4dd*OppF4_3d + zeta_3d*Oppldots_3d + Bz*(2*OppSz_3d + OppLz_3d)+ H112 * (OppSx_3d+OppSy_3d+2*OppSz_3d)/sqrt(6) + tenDq*OpptenDq_3d + tenDqL*OpptenDq_Ld + Veg * OppVeg + Vt2g * OppVt2g + edfinal * OppN_3d + eLfinal * OppN_Ld + epfinal * OppN_2p + zeta_2p * Oppcldots + F0pd * OppUpdF0 + F2pd * OppUpdF2 + G1pd * OppUpdG1 + G3pd * OppUpdG3 -- we now can create the lowest Npsi eigenstates: Npsi=3 -- in order to make sure we have a filling of 8 electrons we need to define some restrictions StartRestrictions = {NF, NB, {"000000 00 1111111111 0000000000",8,8}, {"111111 11 0000000000 1111111111",18,18}} psiList = Eigensystem(Hamiltonian, StartRestrictions, Npsi) oppList={Hamiltonian, OppSsqr, OppLsqr, OppJsqr, OppSx_3d, OppLx_3d, OppSy_3d, OppLy_3d, OppSz_3d, OppLz_3d, Oppldots_3d, OppF2_3d, OppF4_3d, OppNeg_3d, OppNt2g_3d, OppNeg_Ld, OppNt2g_Ld, OppN_3d} -- print of some expectation values print(" # <E> <S^2> <L^2> <J^2> <S_x^3d> <L_x^3d> <S_y^3d> <L_y^3d> <S_z^3d> <L_z^3d> <l.s> <F[2]> <F[4]> <Neg^3d> <Nt2g^3d><Neg^Ld> <Nt2g^Ld><N^3d>"); for i = 1,#psiList do io.write(string.format("%3i ",i)) for j = 1,#oppList do expectationvalue = Chop(psiList[i]*oppList[j]*psiList[i]) io.write(string.format("%8.3f ",expectationvalue)) end io.write("\n") end -- in order to calculate nIXS we need to determine the intensity ratio for the different multipole intensities -- ( see PRL 99, 257401 (2007) for the formalism ) -- in short the A^2 interaction is expanded on spherical harmonics and Bessel functions -- The 3d Wannier functions are expanded on spherical harmonics and a radial wave function -- For the radial wave-function we calculate <R(r) | j_k(q r) | R(r)> -- which defines the transition strength for the multipole of order k -- The radial functions here are calculated for a Ni 2+ atom and stored in the folder NiO_Radial -- more sophisticated methods can be used -- read the radial wave functions -- order of functions -- r 1S 2S 2P 3S 3P 3D file = io.open( "NiO_Radial/RnlNi_Atomic_Hartree_Fock", "r") Rnl = {} for line in file:lines() do RnlLine={} for i in string.gmatch(line, "%S+") do table.insert(RnlLine,i) end table.insert(Rnl,RnlLine) end -- some constants a0 = 0.52917721092 Rydberg = 13.60569253 Hartree = 2*Rydberg -- pd transitions from 2p (index 4 in Rnl) to 3d (index 7 in Rnl) -- <R(r) | j_k(q r) | R(r)> function RjRpd (q) Rj1R = 0 Rj3R = 0 dr = Rnl[3][1]-Rnl[2][1] r0 = Rnl[2][1]-2*dr for ir = 2, #Rnl, 1 do r = r0 + ir * dr Rj1R = Rj1R + Rnl[ir][4] * math.SphericalBesselJ(1,q*r) * Rnl[ir][7] * dr Rj3R = Rj3R + Rnl[ir][4] * math.SphericalBesselJ(3,q*r) * Rnl[ir][7] * dr end return Rj1R, Rj3R end -- the angular part is given as C(theta_q, phi_q)^* C(theta_r, phi_r) -- which is a potential expanded on spherical harmonics function ExpandOnClm(k,theta,phi,scale) ret={} for m=-k, k, 1 do table.insert(ret,{k,m,scale * math.SphericalHarmonicC(k,m,theta,phi)}) end return ret end -- define nIXS transition operators function TnIXS_pd(q, theta, phi) Rj1R, Rj3R = RjRpd(q) k=1 A1 = ExpandOnClm(k, theta, phi, I^k*(2*k+1)*Rj1R) T1 = NewOperator("CF", NF, IndexUp_3d, IndexDn_3d, IndexUp_2p, IndexDn_2p, A1) k=3 A3 = ExpandOnClm(k, theta, phi, I^k*(2*k+1)*Rj3R) T3 = NewOperator("CF", NF, IndexUp_3d, IndexDn_3d, IndexUp_2p, IndexDn_2p, A3) T = T1+T3 T.Chop() return T end -- q in units per a0 (if you want in units per A take 5*a0 to have a q of 5 per A) q=9.0 print("for q=",q," per a0 (",q / a0," per A) The ratio of k=1 and k=3 transition strength is:", RjRpd(q)) -- define some transition operators qtheta=0 qphi=0 Tq001 = TnIXS_pd(q,qtheta,qphi) qtheta=Pi/2 qphi=Pi/4 Tq110 = TnIXS_pd(q,qtheta,qphi) qtheta=math.acos(math.sqrt(1/3)) qphi=Pi/4 Tq111 = TnIXS_pd(q,qtheta,qphi) qtheta=math.acos(math.sqrt(9/14)) qphi=math.acos(math.sqrt(1/5)) Tq123 = TnIXS_pd(q,qtheta,qphi) -- calculate the spectra nIXSSpectra = CreateSpectra(XASHamiltonian, {Tq001, Tq110, Tq111, Tq123}, psiList, {{"Emin",-10}, {"Emax",20}, {"NE",6000}, {"Gamma",1.0}}) -- print the spectra to a file nIXSSpectra.Print({{"file","NiOnIXS_L23.dat"}}); -- a gnuplot script to make the plots gnuplotInput = [[ set autoscale set xtic auto set ytic auto set style line 1 lt 1 lw 1 lc rgb "#FF0000" set style line 2 lt 1 lw 1 lc rgb "#0000FF" set style line 3 lt 1 lw 1 lc rgb "#00C000" set style line 4 lt 1 lw 1 lc rgb "#000000" set style line 5 lt 1 lw 3 lc rgb "#808080" set xlabel "E (eV)" font "Times,12" set ylabel "Intensity (arb. units)" font "Times,12" set out 'NiOnIXS_L23.ps' set size 1.0, 0.3 set terminal postscript portrait enhanced color "Times" 8 energyshift=857.6 plot "NiOnIXS_L23.dat" using ($1+energyshift):(-$9 -$11 -$13 +0.16) title '011' with lines ls 2,\ "NiOnIXS_L23.dat" using ($1+energyshift):(-$15 -$17 -$19 +0.11) title '111' with lines ls 3,\ "NiOnIXS_L23.dat" using ($1+energyshift):(-$21 -$23 -$25 +0.06) title '123' with lines ls 4,\ "NiOnIXS_L23.dat" using ($1+energyshift):(-$3 -$5 -$7 +0.01) title '001' with lines ls 1 ]] -- write the gnuplot script to a file file = io.open("NiOnIXS_L23.gnuplot", "w") file:write(gnuplotInput) file:close() -- call gnuplot to execute the script os.execute("gnuplot NiOnIXS_L23.gnuplot") -- transform to pdf and eps os.execute("ps2pdf NiOnIXS_L23.ps ; ps2eps NiOnIXS_L23.ps ; mv NiOnIXS_L23.eps temp.eps ; eps2eps temp.eps NiOnIXS_L23.eps ; rm temp.eps")
The spectrum produced:
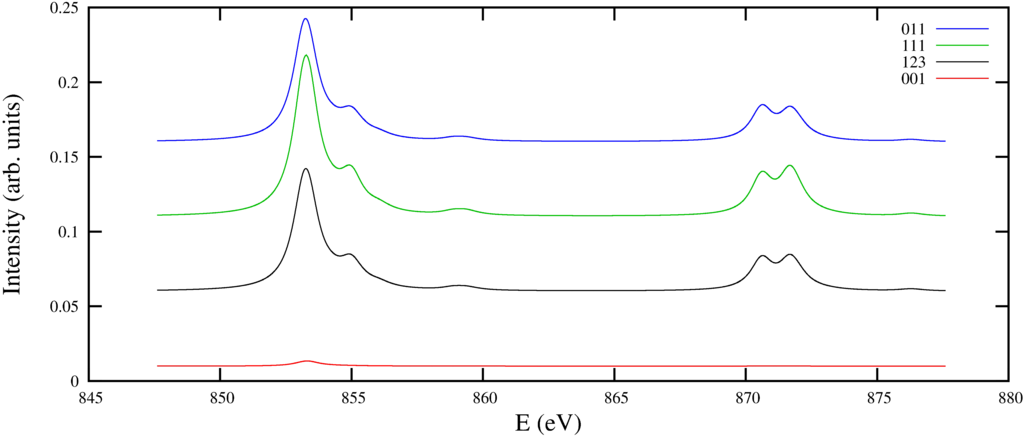 |
| nIXS for NiO looking at a $2p$ to $3d$ excitation |
|---|
The output is to standard out:
# <E> <S^2> <L^2> <J^2> <S_x^3d> <L_x^3d> <S_y^3d> <L_y^3d> <S_z^3d> <L_z^3d> <l.s> <F[2]> <F[4]> <Neg^3d> <Nt2g^3d><Neg^Ld> <Nt2g^Ld><N^3d> 1 -3.395 1.999 12.000 15.147 0.000 0.000 0.000 0.000 -0.905 -0.280 -0.319 -1.043 -0.925 2.189 5.989 3.823 6.000 8.178 2 -3.395 1.999 12.000 15.147 0.000 0.000 0.000 0.000 -0.000 -0.000 -0.319 -1.043 -0.925 2.189 5.989 3.823 6.000 8.178 3 -3.395 1.999 12.000 15.147 0.000 0.000 0.000 0.000 0.905 0.280 -0.319 -1.043 -0.925 2.189 5.989 3.823 6.000 8.178 for q= 9 per a0 ( 17.007535121086 per A) The ratio of k=1 and k=3 transition strength is: 0.081284239649905 0.04426369559805
\lstinputlisting[style=output]{../../Example_and_Testing/History/include/Tutorials/40_NiO_Ligand_Field/49_NIXS_L23.out}